Hashimoto’s disease is an autoimmune disease caused by antibodies that target the tissue of your thyroid gland. It's the most common cause of underactive thyroid in the UK and is more common in women than men. Treatment for Hashimoto’s disease involves replacing the thyroid hormone that your body lacks using medication.
What is Hashimoto’s disease?
Hashimoto’s thyroiditis (chronic lymphocytic thyroiditis) is the most common cause of an underactive thyroid (hypothyroidism) in the UK.
The condition is 7 times more common in women than in men and typically occurs in middle age, though you can develop it at any age.
Hashimoto’s is an autoimmune disease caused by your immune system's production of anti-thyroid peroxidase antibodies (TPOAb) and anti-thyroglobulin antibodies (TgAb).
These antibodies target and confuse the tissue of your thyroid gland, preventing it from producing thyroid hormones, leading to an underactive thyroid.
You can check your thyroid health at home by using a thyroid blood test.
What causes Hashimoto’s disease?
The cause of Hashimoto’s disease is a combination of genetics and non-genetic factors.
The disease has a strong genetic link and tends to run in families — you’re 20 times more likely to have the condition if your sibling is already affected.
Other non-genetic factors that can trigger Hashimoto's disease include:
- having thyroid issues after pregnancy (postpartum thyroiditis)
- excessive iodine intake
- amiodarone — a type of medication used to treat irregular heart rhythms (arrhythmias)
- infections
- radiation to your head or neck
Hashimoto’s disease symptoms
Many people with Hashimoto’s disease show no symptoms at the beginning. But as the disease progresses, you might experience symptoms of an underactive thyroid.
The most common symptoms of underactive thyroid are:
- weight gain
- extreme tiredness
- irregular periods with heavy bleeding (menorrhagia)
- brittle hair or hair loss
- difficulty concentrating
- low mood
- sensitivity to cold
Getting a Hashimoto’s disease diagnosis
Getting a Hashimoto’s disease diagnosis typically involves talking to your doctor about your medical history, as well as a physical examination, and thyroid function blood tests.
Hashimoto’s can also cause a goitre — abnormal swelling of your thyroid gland which might be so large that it’s visible.
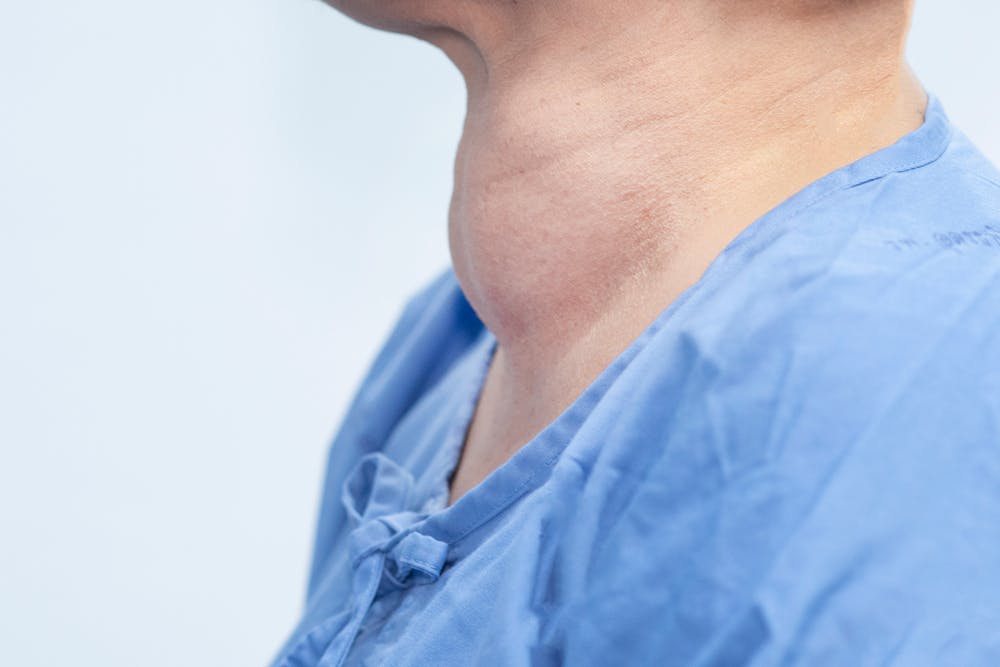
Hashimoto’s thyroiditis is associated with a number of other autoimmune diseases, including:
- type 1 diabetes
- pernicious anaemia
- vitiligo — a condition that causes white patches to develop on your skin, caused by a lack of pigment called melanin
- Addison’s disease — a condition that affects the production of essential hormones in your adrenal glands
- coeliac disease — a condition that causes your immune system to mistakenly attack your tissues when you eat gluten
If you’ve been diagnosed with Hashimoto’s disease, you should be screened for coeliac disease too.
Molecular mimicry theory and Hashimoto’s disease
Several theories explain how your body’s immune system is activated to attack its own thyroid gland. One explanation centres around a concept called ‘molecular mimicry’.
Your immune system is designed to defend your body against potentially dangerous invaders, like viruses or bacteria. These foreign bacteria and viruses contain antigens, which are substances that cause your body to produce antibodies — your immune response. The antibodies attach to the antigens. This attachment is very specific, like a key that only fits only one lock.
Antibodies stay in your body even after the antigen has gone, so they allow your immune system to remember the antigen if it comes back. If the antigen does come back, antibodies can recognise them immediately and mount a much faster and more robust defence against the foreign molecule.
Unfortunately, your immune system’s recognition can sometimes go wrong. If a molecule on the surface of your body’s normal cells very closely resembles a foreign antigen, the antibody might incorrectly lock onto it. This triggers your immune system to attack its own body’s cells rather than the foreign ones that the antibody was originally designed for.
The idea that a foreign antigen can ‘mimic’ a native antigen comes from the term ‘molecular mimicry’.
In the case of Hashimoto’s disease, theories suggest that a primary bacterial infection can cause your body to produce antibodies that go on to attack your thyroid incorrectly.
Hashimoto’s and pregnancy
An untreated underactive thyroid can result in poorer outcomes in pregnancy, including:
- miscarriage
- maternal complications
- abnormal brain development in the foetus
For this reason, pregnant women with known thyroid disease require close monitoring throughout their pregnancy and should take their thyroid medication as prescribed.
What are the treatments for Hashimoto's disease?
Treatment for Hashimoto’s disease involves replacing the thyroid hormone that is lacking using medication.
The most commonly available drug is levothyroxine (LT4). Levothyroxine is a synthetic form of thyroxine (T4) and doesn't include any triiodothyronine (T3). In the same way, thyroxine is converted to T3 in our bodies, levothyroxine is also converted to T3.
There are other drugs used in the treatment of Hashimoto’s that include both T3 and T4. This is known as combination therapy. These drugs aren't generally available on the NHS as there’s insufficient evidence from large-scale studies to suggest that they're superior to levothyroxine alone.
Up to 10% of people with an underactive thyroid still complain of symptoms despite being treated with levothyroxine and having normal levels of their thyroid-stimulating hormone (TSH).
One reason for this, amongst others, could be because levothyroxine isn’t restoring your body’s T4 and T3 levels to normal (physiological) levels.
The European Thyroid Association (ETA) recommends that levothyroxine be the standard therapy for hypothyroidism. But they suggest that combination LT3+LT4 therapy can be used on an experimental basis in patients who still have symptoms despite normal thyroid-stimulating hormone (TSH) levels.
Combination therapy should only be prescribed by a hormone specialist (endocrinologist) with experience in its use.

















